




Ingestion of oral LPS does not cause obesity. A 90-day continuous oral dose study of LPS in rats conducted according to the Organization for Economic Co-operation and Development (OECD) guidelines confirmed that LPS doses up to 45 mg/kg body weight daily did not cause differences from control animals in body weight and did not cause any abnormalities in organs (*1).
In 2007, the "Metabolic endotoxemia initiates obesity and insulin resistance" article was published (*2). Some books cite this article, inadvertently stating that "LPS causes obesity." However, this can lead to misconceptions that LPS present in food, the environment, or the intestines causes obesity and, if this article is to be referenced, there should be a correct and careful explanation accompanying the statement. In this article, it is reported that mice fed a high-fat diet had slightly higher serum LPS. In addition, this article reported that artificial injections of LPS (i.e., intradermal implantation of a device that continuously pumps and injects LPS) show a tendency to cause weight gain and a pattern of inflammation similar to that seen when eating a high-fat diet. In no way does the article state that natural intake of LPS from food or inhaled air would result in obesity. As of 2019, the results of this article can be explained by leaky gut due to a high-fat diet (refer to the section on Leaky gut and LPS). That is, the phenomenon can be explained as follows: if a high-fat diet is consumed, the enteric bacterial balance is disrupted, the intestinal barrier function is broken, enteric bacteria and LPS migrate into the body, and inflammation occurs. Looking at this article, at this time, it seems to be more of a study on leaky gut rather than a study of obesity.
In 2018, a study showing that LPS orally administered to mice on a high-fat die suppress atherosclerosis and Aβ accumulation as well as weight gain was published. (*3, *4).
For those interested in the latest findings on obesity, read the following.
According to the World Health Organization (WHO), the terms overweight and obesity are defined as abnormal or excessive fatty deposits that can impair health (*5). Indicators include the following.
* Body Mass Index (BMI) is a simple measure calculated from the relationship between body weight and height, which is commonly used to classify overweight and obese adults. This is defined as the weight in kilograms divided by the square of the height in meters (kg/m2).
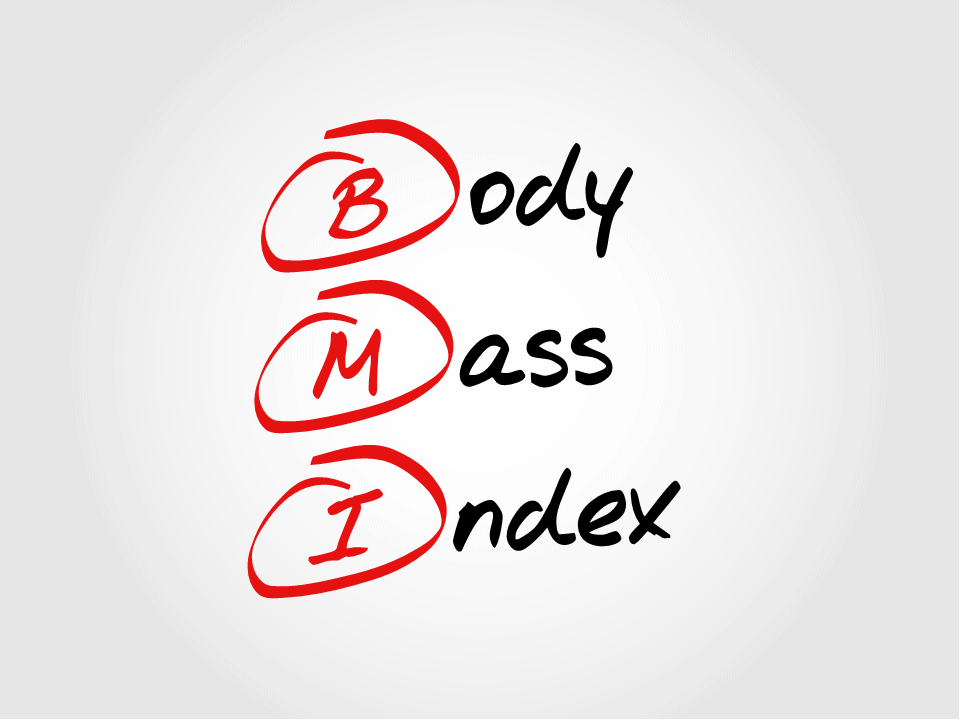
Again, according to the WHO, the underlying cause of obesity and being overweight is a disparity in the energy balance between calories consumed and calories spent (*5). In other words, the cause is excessive caloric intake and lack of exercise.
However, energy imbalance may occur due to genetic factors such as " satiety hormones or metabolic factors do not function normally" (*6). In addition, recent findings suggest that enteric bacterial imbalances may predispose an individual to obesity, as described below.
Several studies have shown a link between obesity and enteric bacteria. For example, in 2013, a paper showing that “the transfer of obese and lean twin enteric bacteria into the intestines of germ-free mice resulted in obesity in mice receiving enteric bacteria from obese individuals and lean mice receiving enteric bacteria from lean individuals” are published (*7). Several studies have also reported that the enteric bacterial balance in obese individuals is different from that of normal individuals (*8). The types of bacteria that increase or decrease are not always consistent, and this is interpreted as obesity being related to a decrease in the diversity of enteric bacteria rather than to a good or bad effect of a particular bacterium.
Why then are enteric bacteria associated with obesity? Enteric bacteria break down dietary fibers that humans cannot break down to produce short-chain fatty acids, such as acetic acid, butyric acid, and propionic acid. These short-chain fatty acids not only serve as an energy source for humans, but also have various functions involved in suppressing obesity as shown below (*9).
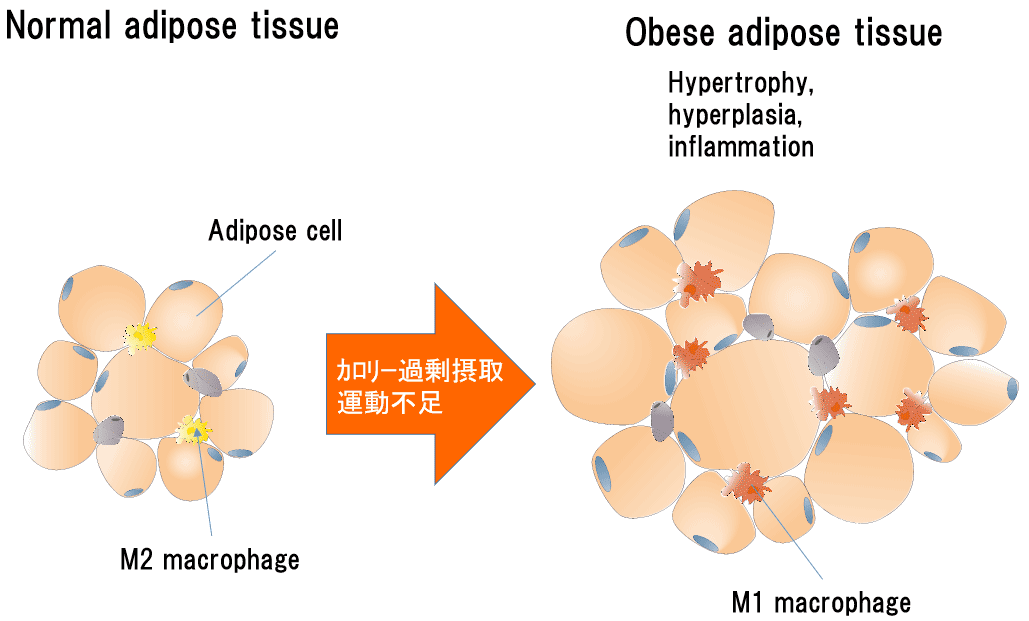
In recent years, obesity has been said to be an inflammatory disease. This is because obesity causes inflammation in adipose tissue.
When adipocytes become hypertrophic as a result of excessive caloric intake, adipocytes begin to secrete factors that recruit macrophages, and both macrophages and adipocytes release inflammatory factors, causing inflammation in adipose tissue (*10). The inflammation cause insulin-resistant (a condition in which cells that receive the signal are less sensitive to insulin, even if insulin is present). If insulin resistance develops, insulin secretion is increased to compensate for its need in effect, resulting in exhaustion or death of pancreatic β-cells, resulting in true insulin deficiency and the onset of type 2 diabetes.
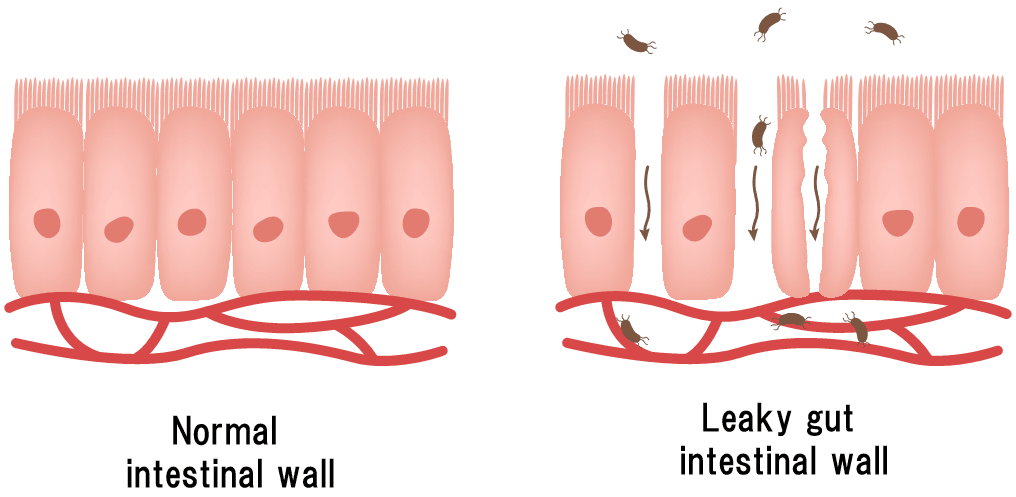
Incidentally, regardless of whether or not an individual is obese, excessive caloric intake can disrupt the balance of enteric bacteria, as described above. Short-chain fatty acids produced by enteric bacteria also increase mucus and anti-inflammatory substances in the intestinal wall. If short-chain fatty acids are reduced due to an imbalance in enteric bacteria, the intestinal barrier function is impaired, leading to leaky gut (*11, *12). If leaky gut occurs in this manner, enteric bacteria and LPS from enteric bacteria migrate into the body and cause inflammation. The reason why obesity is said to be an inflammatory disease seems to be considered together with inflammation via leaky gut as a result of excessive calorie intake.
(*1)in preparation for submission
(*2)Metabolic endotoxemia initiates obesity and insulin resistance.
Diabetes, 56:1761-1772(2007)
(*3)Oral administration of Pantoea agglomerans-derived lipopolysaccharide prevents metabolic dysfunction and Alzheimer's disease-related memory loss in senescence-accelerated prone 8 (SAMP8) mice fed a high-fat diet.
PLOS ONE, https://doi.org/10.137/journal.pone.0198493 (2018)
(*4)Oral administration of Pantoea agglomerans-derived lipopolysaccharide prevents development of atherosclerosis in high-fat diet-fed apoE-deficient mice via ameliorating hyperlipidemia, pro-inflammatory mediators and oxidative resposes.
PLOS ONE, https://doi.org/10.137/journal.pone.0195008 (2018)
(*5)https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
(*6)Obesity – is it a genetic disorder?
Journal of Internal Medicine, 254: 401–425 (2003)
(*7)Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice.
Science, 341:6150-(2013)
(*8)The Gut Microbiome Profile in Obesity: A Systematic Review.
International Journal of Endocrinology, https://doi.org/10.1155/2018/4095789 (2018)
(*9)Diabetes and enteric bacteria
Modern media, 62:159-165 (2016) ; Japanese
(*10)Paracrine regulatory system of adipocytes and macrophages in obese adipose tissue
Obesity Research, 12:70-72 (2006); Japanese
(*11)Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1and E2 production by intestinal myofibroblasts.
Gut, 52: 1442–1447 (2003)
(*12)Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis.
Nature Communications, 9: 3555 (2018)

DynaxT bldg. 2F, 2217-6
Hayashi-cho, Takamatsu-shi,
Kagawa-ken,
761-0301 Japan
TEL : +81-87-867-7712
FAX : +81-87-867-7737
Your personal information on this site is protected by SSL.